
Obesity is increasingly being recognized as a metabolic disorder that accelerates biological aging. Typical consequences of aging such as sarcopenia, atherosclerosis, insulin resistance, and declining adaptive immune function, are aggravated by obesity. Alarmingly, these age-associated health issues are now being observed in younger population, indicating early signs of accelerated aging and raising significant public health concerns.
Recent reviews by two independent research groups have examined the aging hallmarks and their potential links with obesity. Both concluded that the pathophysiological changes in obesity are similar to or may directly contribute to those observed in aging. These include systemic inflammation, telomere shortening, mitochondrial dysfunction, and cellular senescence, among others, suggesting that obesity may accelerate the progressive decline in physiological integrity that typically accompanies aging.
A recent study published in JAMA, Diabetes and Endocrinology assessed young adults with long-term obesity and demonstrated a significant increase in molecular markers of aging, such as elevated high-sensitivity C-reactive protein (hs-CRP), interleukin-6 (IL-6), and apelin, as well as a marked discrepancy between chronological age and biological epigenetic age. Individuals with persistent obesity showed up to a 48% higher biological age than their actual age, along with impaired cardiometabolic function. These findings provide strong evidence supporting the hypothesis that obesity accelerates age-related decline, although the precise molecular mechanisms remain to be fully elucidated (see Graphic).
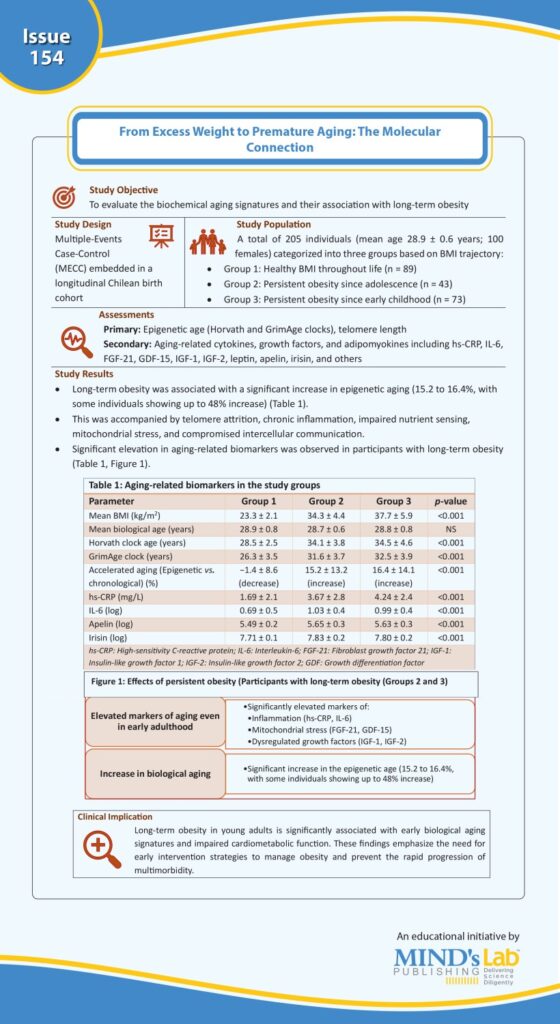
(Source: Correa-Burrows P, Burrows R, Albala C, Sepúlveda C, Salech F, Troncoso R, Bunout D, Gonzalez-Billault C. Long-term obesity and biological aging in young adults. JAMA Netw Open. 2025;8(7):e2520011. Doi:10.1001/jamanetworkopen.2025.20011.)
