India has the highest number of antibiotic fixed-dose combinations (FDCs) in the market, including inappropriate combinations, which are a major concern for an increased risk of antimicrobial resistance. These FDCs have been licensed for manufacturing without the required prior approval of the central regulator and without proper evidence of rationale, efficacy, and safety. To address this problem, the central regulator had taken two major regulatory initiatives (in 2007 and 2013) to remove centrally unapproved systemic antibiotic FDCs from the market.
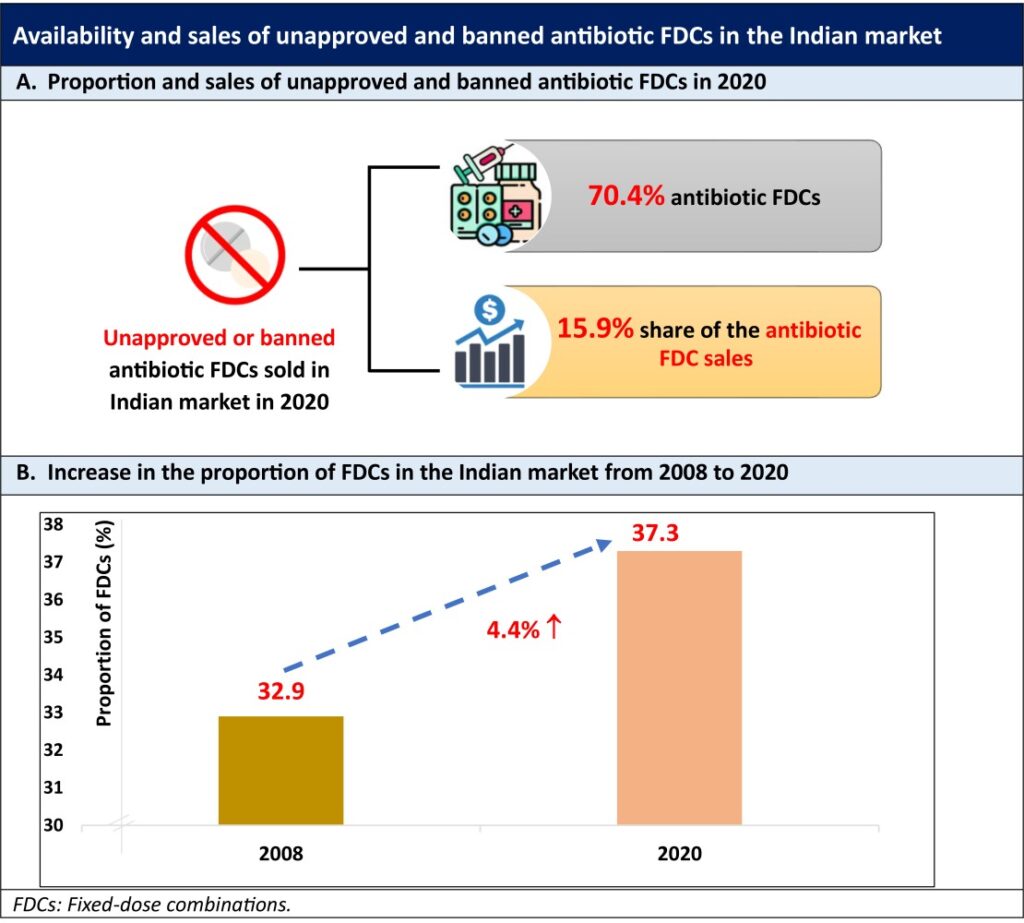
A recent study by Brhlikova P et al., published in the “Journal of Pharmaceutical Policy and Practice”, examined whether the measures taken under the regulatory initiatives were adequate to remove centrally unapproved/irrational systemic antibiotic FDCs from the market. This study reported that most of the antibiotic FDCs (70.4%) sold in India in 2020 were unapproved or banned despite the measures taken under the government initiatives. Moreover, these FDCs accounted for 15.9% share of the antibiotic FDC sales in 2020.
For the study, information was obtained from the official documents published by the central regulator, the Ministry of Health, including the National List of Essential Medicines (NLEM), and court judgments. The annual market sales data of systemic antibiotic FDCs were also tracked from 2008–2020 using the PharmaTrac market dataset and analyzed considering the measures under the initiatives.
The study findings are summarized below:
- A total of 68 systemic antibiotic FDCs were given de facto approval with No Objection Certificates (NOCs) outside the statutory regime, 46 FDCs were banned, and 1 FDC was restricted following the regulatory initiatives.
- The number of formally approved FDC formulations increased from 86 in 2008 to 94 in 2020 and their market share also increased from 32% in 2008 to 55.3% in 2020.
- The number of FDC formulations with NOCs in the market increased from 16 in 2015 to 23 in 2020. Their market share by volume also increased from 8% in 2015 to 10.6% in 2020.
- The market share by volume of NLEM-listed FDCs increased from 21.2% to 26.7% during the study period (2008–2020).
- Although the total number of antibiotic FDCs in the market declined from 574 in 2008 to 395 in 2020, most of the marketed formulations (278/395) were unapproved or banned and accounted for a 15.9% share of the antibiotic FDC sales (Graphic A).
- There was a 4.4% increase in the proportion of FDCs in the market from 2008 to 2020 (Graphic B), with 1.5% increase in single drug volumes.
- A total of 700 million antibiotic FDCs were unapproved of the 4.5 billion standard units sold in 2020. Moreover, an additional one-third of standard units (1.5/4.5 billion) sold in 2020 were of antibiotic FDCs approved in India, but not recommended by the WHO.
Clinical implications
- This study highlights the need to plan a common framework, with coordinated efforts from the state and central regulatory agencies to curb the availability of irrational, unapproved, and banned FDCs of antibiotics in the Indian market.
- This will enable rational prescription of FDCs by the doctors, a measure that is extremely important in view of the rise in antimicrobial resistance across the world.
- Regular surveillance of the market will help in assessing the implementation of the policies and adoption of antimicrobial stewardship.
(Source: Brhlikova P, Mehta A, McGettigan P, Pollock AM, Roderick P, Farooqui HH. Regulatory enforcement of the marketing of fixed-dose combinations in India: A case study of systemic antibiotics. Journal of Pharmaceutical Policy and Practice. 2023;16(1):139. Doi: 10.1186/s40545-023-00644-y)
