
Antimicrobial resistance (AMR) is increasing every year at an alarming rate, posing a serious global health threat and endangering people’s lives [1]. India is too grappling with high AMR rates [2]. One of the key factors for high AMR in India (Figure 1) is high antibiotic consumption rate, which is 10.7 units (i.e., pill, capsule, or ampoule) per person. In view of the rising resistance trends, clinicians resort to the use of broad-spectrum antibiotics, which increases their consumption rate, thereby limiting treatment choices and impacting clinical outcomes. Thus, effective antimicrobial strategies for drug-resistant organisms are the need of the hour [1].
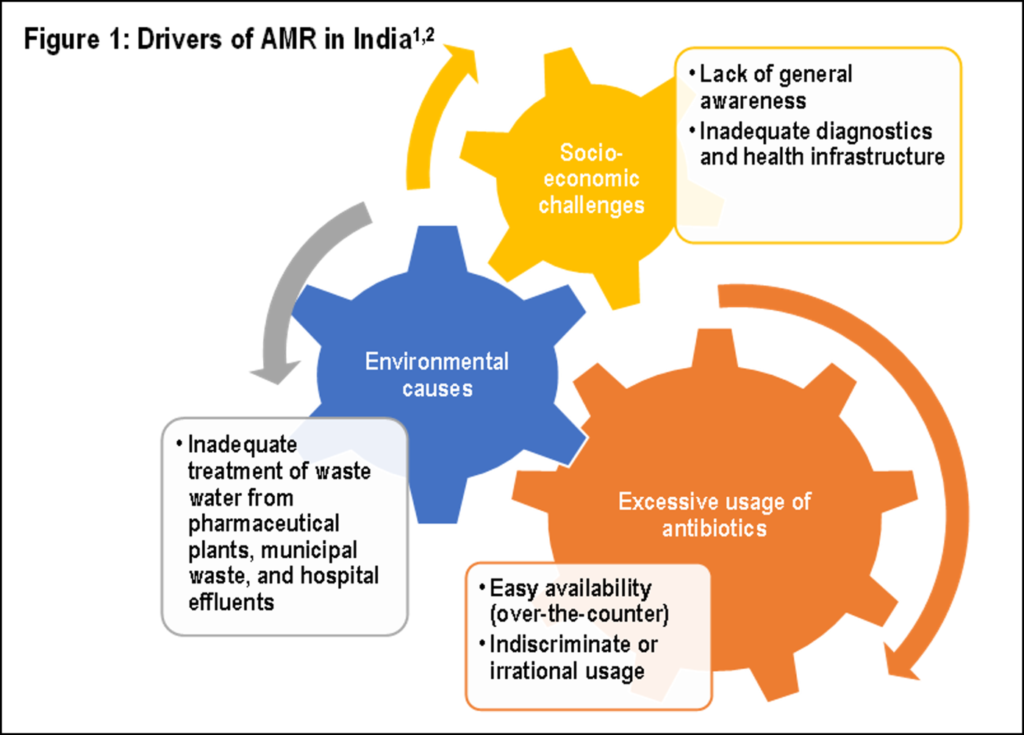
Current AMR trends in India
In India, higher rates of multi-drug resistance are observed in Gram-negative organisms compared to Gram-positive organisms, attributed to the development of various drug resistance mechanisms, particularly among Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa [2,3]. The latest 2021 report of Indian Council of Medical Research on AMR highlighted increasing prevalence of multidrug resistant (MDR) Gram-negative pathogens, showing very low susceptibility rates of fluoroquinolones and third-generation cephalosporins, and increasing rates of carbapenem resistance (see the graphic below) [4].
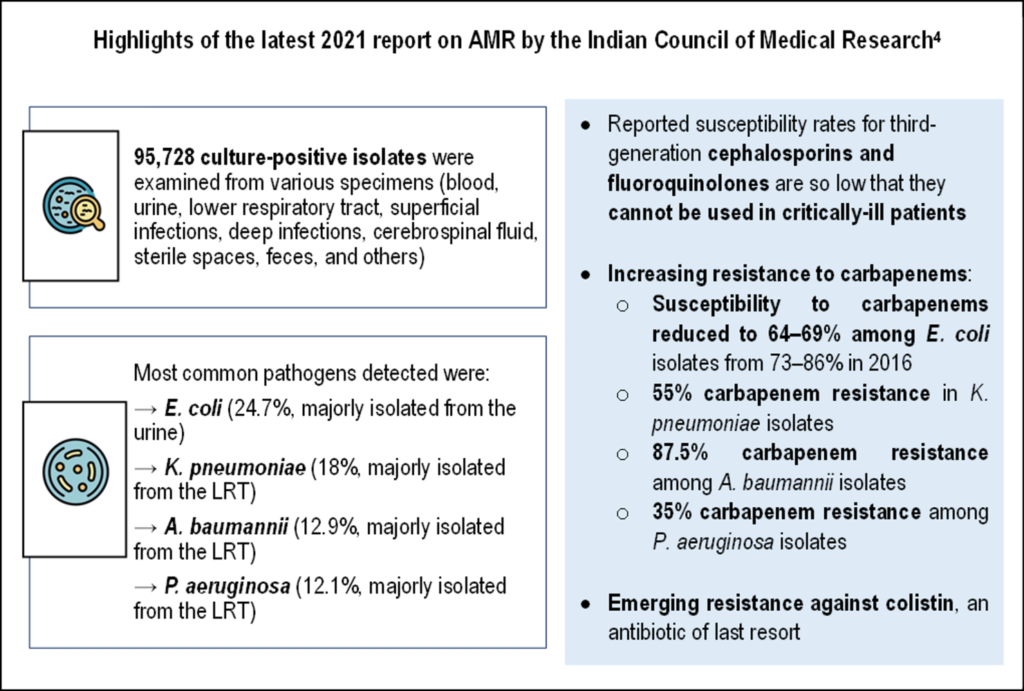
WCK 5222: A potential therapy against multi-drug resistant infections
Resistance to available antimicrobials, including carbapenems, limits the scope for treating multi-drug resistant infections, thus creating a huge treatment gap. To address this unmet need in treating resistant bacterial infections, a novel drug, WCK 5222, is currently being developed in India[5,6].
WCK 5222 is a combination of a β-lactam antibiotic, cefepime, and a β-lactamase inhibitor, zidebactam. The combination has shown potent activity against multi-drug resistant, β-lactamase-producing Gram-negative pathogens. Zidebactam, a first-in-class β-lactam enhancer, inhibits a range of β-lactamases (Class A–D). Thus, the novel antibiotic with the universal stability of zidebactam against almost all β-lactamases and synergistic action of cefepime may be a potential treatment against infections caused by MDR Gram-negative bacteria (refer to the graphic below) [5-8].
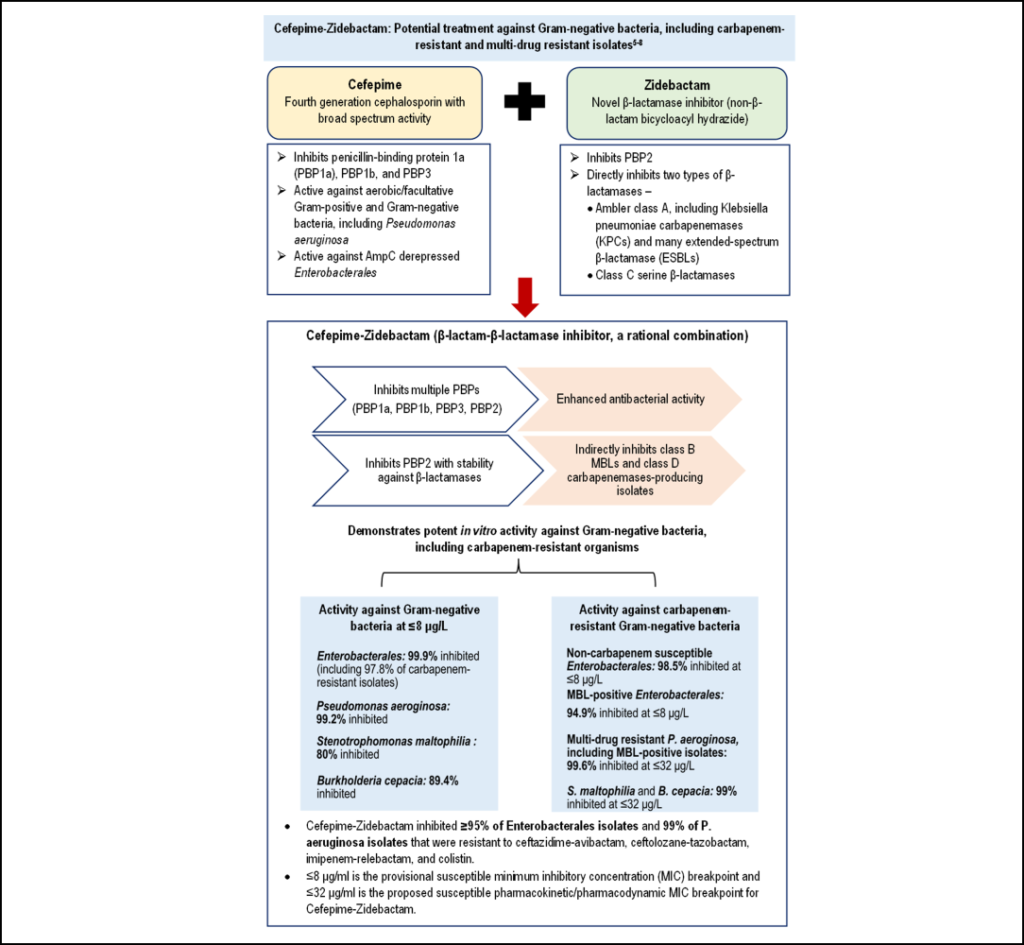
Clinical implications: Rising prevalence of resistant infections poses a significant challenge to the healthcare system. With limited treatment options to treat drug-resistant infections, an increase in the healthcare burden, including medical costs, hospitalizations, and morbidity and mortality, is evident [1-3]. To tackle this overwhelming situation, it is necessary to adopt judicious use of antibiotics, along with developing more potent line of attack for resistant infections. A novel drug, WCK 5222 (cefepime/zidebactam), a combination of a β-lactam and a β-lactamase inhibitor, has demonstrated potent inhibitory activity against β-lactamases-producing Gram-negative pathogens, including P. aeruginosa and A. baumannii, that have shown resistance to almost all currently marketed broad-spectrum antibiotics [5-8]. The highly anticipated novel antibiotic, scheduled to enter Phase III clinical trials in India, may be the future treatment of choice for multi-drug resistant infections.
The World Antimicrobial Awareness Week (18–24 November, 2022) is a global campaign to improve awareness and understanding of antimicrobial resistance (AMR) and encourage best clinical practices. This year’s theme is ‘Preventing Antimicrobial Resistance Together’ calling for judicious use of antimicrobials and adopting effective preventive measures to tackle AMR in a collaborative approach.
References
1. Manesh A, Varghese GM; CENDRIC Investigators and Collaborators. Rising antimicrobial resistance: An evolving epidemic in a pandemic. Lancet Microbe. 2021;2(9):e419-20.
2. Taneja N, Sharma M. Antimicrobial resistance in the environment: The Indian scenario. Indian J Med Res. 2019;149(2):119-28.
3. Veeraraghavan B, Walia K. Antimicrobial susceptibility profile & resistance mechanisms of Global Antimicrobial Resistance Surveillance System (GLASS) priority pathogens from India. Indian J Med Res. 2019;149(2):87-96.
4. Annual Report 2021. Antimicrobial Resistance Research and Surveillance Network. Division of Epidemiology and Communicable Diseases, Indian Council of Medical Research (ICMR) [Internet]. 2021 [cited 2022 Nov 15]. Available from Annual Report 2021: Antimicrobial Resistance Research and Surveillance Network (icmr.nic.in)
5. Karlowsky JA, Hackel MA, Bouchillon SK, et al. In vitro activity of WCK 5222 (cefepime-zidebactam) against worldwide collected Gram-negative bacilli not susceptible to carbapenems. Antimicrob Agents Chemother. 2020;64(12):e01432-20.
6. New drug discovery, Wockhardt [Internet]. 2016 [cited Nov 16]. Available from New Drug Discovery (wockhardt.com)
7. Pan X, Zhao X, Song Y, et al. Molecular characterization of WCK 5222 (cefepime/zidebactam)-resistant mutants developed from a carbapenem-resistant Pseudomonas aeruginosa clinical isolate. Microbiol Spectr. 2022;10(1):e0267821. Doi: 10.1128/spectrum.02678-21
8. Sader HS, Mendes RE, Duncan LR, et al. Antimicrobial activity of cefepime/zidebactam (WCK 5222), a β-lactam/β-lactam enhancer combination, against clinical isolates of Gram-negative bacteria collected worldwide (2018-19). J Antimicrob Chemother. 2022;77(10):2642-49.
